Introduction
Despite all the therapeutic progress made in multiple myeloma (MM), the standard of care for eligible patients remains an intensive induction therapy followed by high dose melphalan and autologous stem cell transplantation (ASCT).
Material and methods
This present retrospective study included MM patients treated in Lyon between 1997 and 2020. All patients received induction chemotherapy followed by intensive chemotherapy and ASCT, with or without consolidation and maintenance therapy. Number and type of therapeutic lines administered for relapse after ASCT were analyzed. All data were reported in the European blood and marrow transplantation (EBMT) PROMISE registry.
Statistical analysis
Descriptive analyses were used for demographic variables, biological parameters, and treatment characteristics. Overall survival (OS) and progression-free survival (PFS) were presented using the Kaplan-Meier method and log-rank tests were performed to compare survival curves. Univariate and multivariate analyses were computed using Cox regressions and the multivariate analyses considered the variables for which the univariate ANOVA p-value was≤ 10%. Statistical analyses and graphics were computed with R v4.1.2, with the help of ‘survival’ and ‘ggplot2‘ packages.
Results
Before ASCT
A total of 342 patients were included in the study (median 60 years (range: 28-73), 58% were male, 89.4% were diagnosed with a type III myeloma according to the Salmon and Durie classification. No genetic analysis was performed at that time. Most patients (92.5%) had a performance status (PS) of 0 or 1. The type of myeloma was heavy chain and light chain myeloma for 75.7% patients (68.9% of IgG kappa) and light chain myeloma in 23.1%. The median delay between diagnosis and ASCT was 4.8 months. Before ASCT, patients received an induction therapy with VTD in 54.1% of cases, VD in 30.1%, and more recently VRD in 16.1%. Disease status before ASCT was complete remission (CR) for 17.5% of the patients, partial remission (PR) and very good partial remission (VGPR) for 79.2% of patients and progressive disease or relapse for 3.0% of them.
After ASCT
The median duration of aplasia was 11 days. At day 100, the response was CR in 166 patients (50.5%), 182 patients (54.7%) received a consolidation treatment and 71 patients (20.8%) a maintenance therapy (Revlimid® for 90% of them). Overall, 227 (66.4%) patients relapsed. The median delay between ASCT and relapse was 22.5 months (0.89-229.13) and the median follow-up duration was 46.7 months (0.33-287.97). Among the 227 patients who relapsed, 6 patients were not treated, 79 patients (23.1%) received one chemotherapy line, 32 patients (9.4%) received two lines, 38 (11.1%) received 3 and 72 (21.1%) received at least 4 lines and up to 8. Overall survival and progression free survival
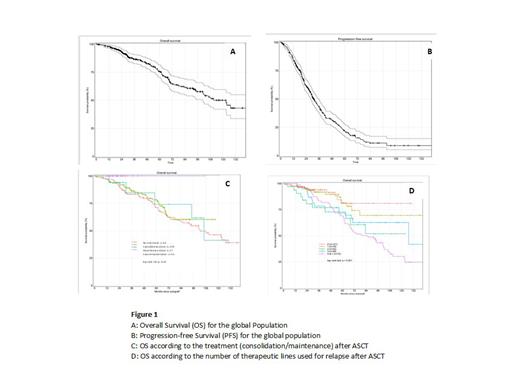
The median OS was 132 [95% CI 110.6; NR] months from diagnosis and 122.8 [100.9; NR] months from ASCT. The median PFS was 39.35 [36.25; 43.37] months from diagnosis and 31.07 [28.42; 35.91] months from ASCT (Figure 1 A, B). In multivariate analysis on OS, the shorter aplasia duration (p<0.001) and consolidation and maintenance (p<0.001) were significantly associated with a significant better OS. The median OS was not reached from ASCT for patients who have received both consolidation and maintenance treatments and no death was reported. (Figure 1 C). For patients receiving chemotherapy lines for relapses after ASCT (Figure 1D) regardless of the number of lines the median OS was102.4 months and the 10-year OS probability was 45.6%. The OS of patients who received 3 lines of treatment was identical to that of patients who received one or 2 lines after ASCT. The median OS after 1 to 2 lines of chemotherapy after ASCT was not reached and was 127.15 months [70.6-NR] for those receiving 3 lines (Figure 4B). The OS of patients who received more than 4 chemotherapy lines after ASCT was significantly lower compared to the OS (p=0.0096) with a median [95%CI] OS of 85.5 months [65.61-106.15].
In conclusion this study showed the significant positive impact on OS of the association consolidation and maintenance. We have also studied the impact of number of chemotherapy lines and the impact on OS and PFS of kind of treatment will be discussed.
Disclosures
Guillermin:Pfizer: Consultancy.